Suche
Lesesoftware
Info / Kontakt
Bitterness - Perception, Chemistry and Food Processing
von: Michel Aliani, Michael N. A. Eskin
Wiley-Blackwell, 2017
ISBN: 9781118590232 , 264 Seiten
Format: ePUB
Kopierschutz: DRM




Preis: 176,99 EUR
eBook anfordern 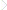
Chapter 1
Biochemistry of Human Bitter Taste Receptors
Jasbir Upadhyaya, Nisha Singh, Raj Bhullar and Prashen Chelikani
1.1 Introduction
The gustatory system has been selected during evolution to detect nutritive and beneficial compounds as well as harmful substances. Humans, and probably other mammals, can taste many compounds but distinguish between five basic tastes which are sweet, bitter, sour, salt and umami. Sour and salt tastes are thought to be perceived via cation channels (Heck et al., 1984; Kinnamon et al., 1988; Ugawa et al., 1998). In contrast, sensation of bitter, sweet and umami tastes is initiated by the interaction of taste molecules with G protein-coupled receptors (GPCRs) (Adler et al., 2000; Gilbertson et al., 2000; Sainz et al., 2001). Bitter taste, among all tastes, is believed to have evolved as a central warning signal against the ingestion of potentially toxic substances. The molecular events in the perception of taste start at the apical surface of taste receptor cells (TRCs) found in taste buds in the mouth. Taste buds are found in taste papillae located on the tongue, the palate, and to a lesser extent the epiglottis, pharynx and larynx, and each taste bud is formed of 50-100 TRCs (Lalonde and Eglitis, 1961; Miller, 1986; Brouwer and Wiersma, 1978). The interaction of tastants with taste receptors, located in the membrane of TRCs, initiates signaling cascades which are transmitted to the brain through sensory afferents and perceived as taste (Chen et al., 2011).
1.2 Bitter Taste Receptors: T2Rs
In humans, bitter taste is perceived by 25 members of the GPCR superfamily, referred to as T2Rs, which are 291 to 334 amino acids long (Adler et al., 2000, Chandrashekar et al., 2000, Matsunami et al., 2000). These taste receptors, discovered a little more than a decade ago, encode for intronless genes which are referred to as TAS2Rs. The HUGO gene nomenclature of TAS2R is used wherever the gene is mentioned. Except for the TAS2R1 gene, which is localized on chromosome 5p, all other TAS2Rs are organized in the genome in clusters on human chromosomes 7q and 12p, and are genetically linked to loci that influence bitter perception (Conte et al., 2002). Additionally, there are a large number of TAS2R pseudogenes and more than 80 single nucleotide polymorphisms (SNPs) among individual TAS2R genes (Conte et al., 2002; Kim et al., 2005). The classification of T2Rs within the GPCR family is unclear, with some describing them as a separate family (Horn et al., 2003), whereas other classification systems have grouped them with the frizzled receptors (Fredriksson et al., 2003). The International Union of Basic and Clinical Pharmacology (IUPHAR) list Frizzled receptors as a separate GPCR family, Class F, and this class does not include T2Rs (Sharman et al., 2013). T2Rs are relatively divergent, showing ∼25–90% amino acid identity (Adler et al., 2000; Matsunami et al., 2000). This variability corresponds well with an ability to interact with chemically diverse ligands associated with bitter tastes. A single bitter compound is capable of activating multiple T2Rs and each T2R can be activated by multiple bitter compounds (Meyerhof et al., 2010). Like all GPCRs, T2Rs contain seven transmembranes (TMs), three extracellular loops (ECLs) and three intracellular loops (ICLs), with a short extracellular N- and an intracellular C-terminus (Fig. 1.1). The other class of taste GPCRs, which codes for sweet and umami receptors (T1Rs), belongs to the class C GPCR family (Lagerstrom and Schioth, 2008). Sweet and umami tastes are mediated by three GPCRs that combine to form two heterodimeric receptors, T1R1/T1R3 for umami and T1R2/T1R3 for sweet-tasting compounds (Li et al., 2002; Nelson et al., 2001, 2002; Zhao et al., 2003). In contrast to the short N-terminus of T2Rs, T1Rs are characterized by a long N-terminus, also known as Venus flytrap, which forms the primary or orthosteric ligand binding site (Pin et al., 2003). Differences in ligand specificity between species has been reported for the sweet and umami receptors (Xu et al., 2004; Li et al., 2002; Nelson et al., 2002). Human T1R1/T1R3 specifically responds to L-Glu, whereas mouse T1R1/T1R3 responds more strongly to other L-amino acids than to L-Glu. In a recent study, the residues in the extracellular Venus flytrap domain of T1R1 which are crucial for amino acid recognition in the human- and mouse-type responses were identified (Toda et al., 2013). In contrast to the low amino acid identity in the N- and C-termini and the ECLs, sequence conservation is more in the TMs and ICLs of T2Rs. The TMs and ECLs are the predicted regions of ligand binding in T2Rs and ICLs are the regions for G-protein interaction (Adler et al., 2000).
Figure 1.1 Predicted secondary structure model of the bitter taste receptor T2R4. The coding region is 299 amino acids long, has a short extracellular N-terminus, three extracellular loops, seven transmembrane (TM1-TM7) helices, three intracellular loops and a short C-terminus.
1.3 T2R Signal Transduction
Long before the discovery of T2Rs, the involvement of taste-specific Gα protein, Gα-gustducin, in bitter receptor mediated transduction mechanism was demonstrated (Wong et al., 1996). The generation of α-gustducin knock-out mice resulted in dramatic reduction of their bitter tasting abilities. Moreover, T2Rs were shown to functionally couple to transducin (He et al., 2002) in vivo as well as to other Gi/Go proteins in vitro (Ozeck et al., 2004). The mechanism involved in the perception of bitter taste and the second messengers or other downstream components of T2R signaling pathway were also known before the T2Rs were discovered in 2000 (Kurihara et al., 1994; Spielman et al., 1996; Chandrashekar et al., 2000). A cation channel, transient receptor potential melastatin subtype 5 channel (TRPM5), was found coexpressed with other taste signaling molecules in taste tissue (Perez et al., 2002).
The canonical T2R signal transduction pathway is described below. The binding of a bitter-tasting compound, also referred to as an agonist, on the extracellular surface of a T2R causes conformational changes in the receptor, and this in turn activates the heterotrimeric G-protein complex, α-gustducin, β1/3 and γ13 on the intracellular surface of the receptor. The βγ-subunits activate the enzyme phospholipase Cβ2 (PLC β2) which hydrolyzes inositol phospholipid (PIP2) resulting in the production of 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Generation of IP3 activates IP3 receptors on the membrane of endoplasmic reticulum (ER), thus opening the calcium release channels and causing transient increase in intracellular calcium. This opens the monovalent selective TRPM5 channels, leading to sodium influx, membrane depolarization and thus release of ATP as a neurotransmitter to activate the gustatory afferents (Finger et al., 2005) (Fig. 1.2). Gα-gustducin activates phosphodiesterases (PDEs) which lead to a reduction in cAMP production (McLaughlin et al., 1992; Spielman, 1998).
Figure 1.2 Bitter taste signaling pathway (IP3 pathway) Abbreviations: PLCβ2, phospholipase C β2; PIP2, phospatidyl-inositol-biphosphate; DAG, diacylglycerol; IP3, inositol triphosphate; ER, endoplasmic reticulum; Ca2+, calcium; Na+, sodium.
1.4 Bitter Taste Perception and T2R Polymorphisms
The sensitivity of humans to the perception of some bitter compounds varies greatly (Bartoshuk, 2000a, 2000b). This variable bitter taste perception is the best-known example of genetic variation in oral sensation. A vast number of structurally diverse compounds elicit bitter taste in humans and many bitter substances can be detected at concentrations roughly 1000-fold lower than substances that stimulate other basic tastes (Meyerhof, 2005). Studies on the genetics of taste perception for phenylthiocarbamide (PTC) began in the early 1930s with the accidental finding by A. L. Fox that crystals of PTC tasted very bitter to some people but not to others (Fox, 1932). Thus, 6-n-propyl-2-thiouracil (PROP) and PTC, which share thiocyanate (NC=S) moiety, taste bitter to some people but are tasteless to others (Fox, 1932).
Sensitivity to PTC/PROP is an inherited trait, and PROP sensitivity was linked with lower acceptability of other bitter compounds and lower reported liking for some bitter foods. Based on the detection thresholds for PTC/PROP solutions, people were categorized into supertasters, tasters and non-tasters. Similarly, inbred mouse strains differ in their ability to detect certain bitter taste stimuli, such as sucrose octaacetate (SOA) and cycloheximide. Genetic studies in humans have demonstrated that the ability to detect PROP is determined by a locus on chromosome 5p15 (Reed et al., 1999).
How humans respond to different bitter tasting compounds is an important question in the field of bitter taste research. Missense mutations were found in the sequences of T2R5 in mouse strains deficient to cycloheximide sensitivity (Chandrashekar et al., 2000). These genetic variants, found in bitter-insensitive mouse strains, also were less responsive in cell-based assays compared with alleles from bitter-sensitive strains. This demonstrated that alleles of a taste receptor can change both behavioral and cellular...





