Suche
Lesesoftware
Info / Kontakt
Surface Modification of Polymers - Methods and Applications
von: Jean Pinson, Damien Thiry
Wiley-VCH, 2019
ISBN: 9783527819232 , 460 Seiten
Format: ePUB
Kopierschutz: DRM




Preis: 142,99 EUR
eBook anfordern 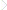
1
The Surface of Polymers
Rosica Mincheva and Jean‐Marie Raquez
University of Mons (UMons), Laboratory of Polymeric and Composite Materials, Center of Innovation and Research in Materials and Polymers (CIRMAP), 20, Place du Parc, 7000 Mons, Belgium
1.1 Introduction
Surface properties of any polymers have an imminent influence over key properties such as wetting, adhesion, friction, and biocompatibility, therefore affecting the applicability of a polymer material [1]. It is nowadays well accepted that surface properties differ from bulk in many aspects and a multitude of scientific works has been done for the last 70 years in an attempt to highlight what actually constitutes the surface, including the interphase, and how far into the material its surface goes [2–10]. Moreover, while classical surface model will consider a surface as rigid, immobile, and at equilibrium, which is more likely to be true for rigid solids, the surface of a polymer material is highly depending on time and temperature due to its viscoelastic behavior and is therefore thermodynamically and kinetically dependent [11]. From this viewpoint, the polymer surface can continuously restructure and reorient in response to different external factors such as atmosphere, solvent, and so on and might be inherently a nonequilibrium dynamic system. The guiding force for these structural changes is that the surface tends to decrease its free energy in a continuous way. In other terms, surface chemistry, reactivity, and aspect vary in function of environmental and processing conditions, influencing any desired modification and/or application of the related material even when bulk properties are considered [12].
In order to understand the application‐related modification of a polymer surface, one should first learn what the polymer surface is actually, how its properties are generally influenced, and what analytical methods are the most appropriate to study and understand. This chapter aims at providing a summary of experimental and theoretical concepts describing polymer surfaces near interfaces. It discusses the role of the different factors such as the surrounding environment in the surface properties and shows the multitude of analytical tools under different situations involving surfaces and interfaces.
1.2 The Surface of Polymers
1.2.1 Definition of a Polymer Surface
The word “surface” in its most general use includes the outermost or the uppermost layer/boundary of a physical object or space/area (http://www.wordreference.com/definition/surface). From the materials science viewpoint, the surface, defined as the frontier between two different media, is characterized by a certain thickness, reflecting a gradient of properties. With this respect, surface ever differs from the bulk of any material in terms of density, composition, or structure, and, even if it is present at very small fraction (by comparison to bulk), the surface governs any polymer properties, as being the first contact sets on. This statement remains true whatever the macroscopic material, including polycrystalline solids or polymers.
However, for polymer surfaces, the molecular length scale goes well above the angstrom scale (e.g. a typical end‐to‐end distance is about 10−6 m for a polymer of 10 000 monomer units and considering the random‐coil conformation [13]), and the term “small fraction” is broadly true. Herein, the connectivity, the entanglements and the interactions between polymer chains at the surface are built up for a surface thickness varying from several nanometers (for a layer in direct contact with other medium) up to several micrometers (for a crystalline morphology) [12]. Even though the interactions decrease upon increasing the distance, they remain the source of cohesion and determine the surface properties such as friction, adhesion, surface tension, and biological activity. Moreover, polymer chains have high degree of freedom (side‐group [methyl, hydroxyl, carbonyl, etc.] C–C rotation, segmental α‐process, and overall chain dynamics) and actual time and temperature‐dependent local or long‐range motions, making surfaces dynamic objects thereof [14] undergoing rearrangements upon changes into the surrounding phase(s): gas(es), liquid(s), or solid(s). Additionally, for a polymer macromolecule in the bulk, the interactions are similar in terms of type and force in all three directions, while for a macromolecule at the surface, they are unbalanced, leading to an excess of surface/interface free energy [15]. All these characteristics create a thermodynamic force (configurational entropy) – the guiding force determining an equilibrium state of minimal free energy or of maximum entropy by transferring end‐groups, functional groups, or additives to the surface, which on the other hand causes segregation of polymer chains and/or their parts [5,12]. The phenomena are known since the 70th of the twentieth century [16] and are emphasized even today [17]. Examples can be found for gels (presenting low or high contact angle in contact with water or air, respectively [18]), grafted polymers (where the grafted chains are found to be hidden in the bulk or exposed on the surface depending on the treatment conditions [19]), or even segmented polymers [16].
Consequently, a polymer surface is a dynamic surface having temperature and environmental responses – a place where phenomena provoking major evolutions influence the polymer properties and lifetime (Figure 1.1) [12,20].
Figure 1.1 Schematic representation of factors determining a polymer surface and its related properties.
1.2.2 Factors Determining a Polymer Surface
The previously driven consideration suggests that the polymer surface will be determined by a multitude of factors within complex relationship without a sharp discrepancy between them. A very general classification, however, can be done based on factors' origin: (i) internal – related to the polymer itself and (ii) external – environment related.
1.2.2.1 Internal Factors
Among the internal factors, the polymer chemistry, composition and structure, and molar mass and dispersity can be listed:
Polymer Chemistry
It is generally considered that aliphatic CC or CO bonds with non‐bulky substituents are quite mobile and flexible, which make them able to adopt any infinite number of configurations (in the ideal case) with quasi‐equal energy and to have a maximum entropy at thermodynamic equilibrium. In this case, the substituents will be exposed to the surface or not depending on the environment as shown by Cimatu et al. [21] for substituted (in terms of ethyl/methyl groups) polymethacrylates with hydroxyl, chloro, or phenoxy moieties. On the other hand, cyclic aliphatic or aromatic structures, branches, or cross‐linking points, as bulky substituents have a marked stiffening effect that forces polymer chains to adopt a certain configuration that will reduce system entropy and increase free energy [22]. Bulky substituents will therefore be segregated at the surface [23], as illustrated by the studies of Hirai et al. [24] on polymethacrylates with “side crystalline” chains. Restrictive chain mobility and conformation are also related with the presence of functionalities allowing attractive hydrogen, dipole, or electrostatic interactions. Such functionalities will force adopting a certain conformation out of thermodynamic equilibrium. In other words, chemistry determines mobility and the properties of a dynamic polymer surface such as surface topography (or roughness) from atomic to macroscopic scale and surface morphology in terms of crystallinity and crystal structure [12,13,25]. Together, polymer chemistry, mobility, and conformation will influence the surface chemistry and thus the surface topography and wetting. Therefore, they all will play on surface mechanical properties, adhesion, friction, etc.
Polymer Composition and Structure
Additives in polymers, especially of low molar mass (e.g. plasticizers), are often excluded from polymer bulk and migrate to the surface, changing its properties and composition [2]. Moreover, the composition will change with thickness and form a gradient. This is similar in the case of immiscible polymer blends, where the blend component with lower surface free energy will migrate to the polymer surface in order to reduce the total free energy of the system and place it in a thermodynamic equilibrium. Surface excess and concentration gradient can be calculated based on the mean field arguments [26,27].
A similar effect is observed with block copolymers or comb‐type copolymers where one sequence enriches the surface depending on miscibility and composition. In some particular cases, such segregation may even lead to the formation of lamellar structure normal to the surface [2].
Molar Mass and Dispersity
Other important factors influencing polymer surfaces are polymer molar mass and dispersity [22]. A low molar mass polymer or a polymer with large dispersity, for example, is expected to present a greater number of chain ends at the polymer surface, when comparing with...





